Reference No.: 808154-1
FOR IN VITRO DIAGNOSTIC USE
This instruction for use (IFU) must be read carefully prior to use. Instruction for use must be carefully followed. Reliability of assay results cannot be guaranteed if there are any deviations from the instructions for use.
The test is for Professional Use Only.
PACKING SPECIFICATION
20 Tests/ Kit
INTENDED USE
This kit is used for in vitro qualitative detection of SARS-CoV-2 antigen, influenza A virus antigen and influenza B virus antigen in human nasopharyngeal swab and oropharyngeal swab samples.
PRINCIPLE OF THE PROCEDURE
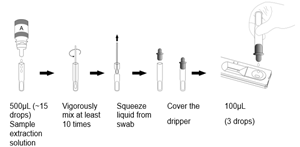
Based on immunochromatography assay technology, the kit uses double antibody sandwich method to qualitatively detect SARS-CoV-2 antigen and Influenza A/B virus antigen in human nasopharyngeal swab and oropharyngeal swab samples.
Because the three test items (SARS-CoV-2 antigen detection, influenza A virus antigen detection and influenza B virus antigen detection) of the test card adopt the parallel mode of independent channels, testing a single item alone will not affect the testing of other items. After aligning the test card, from left to right, there are SARS-CoV-2 antigen test item (SARS-CoV-2 Ag), influenza A virus antigen test item (InFlu A Ag), and influenza B virus antigen test item (InFlu B Ag).
When testing, if the sample to be tested contains the test item antigen, the test item antibody labeled with latex will bind to the antigen in the sample to form a reaction complex. Under the action of chromatography, the reaction complex moves forward along the nitrocellulose membrane, forming a double antibody sandwich “Latex-Ab-Ag-Ab" with antibody of the test item pre-coated in the test zone (T), and finally a red reaction line appears in the test zone. On the contrary, when a negative sample is tested, no red reaction line is formed in the test zone. No matter whether the sample to be tested contains the test item antigen, the quality control zone (C) will always form a red reaction line.